Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose : The role of combined treatment with csDMARDs and anti-TNF therapy in AS is not well established.
Methods: Main goal of this study was to compare the probability of achieving low disease activity (LDA) while persisting on adalimumab (ADA) therapy in AS patients treated by either ADA alone, or by combination of ADA + csDMARDs. This analysis was conducted within the national biologics registry with mandatory registration for all patients with AS who start treatment with biologics. All patients with AS treated with ADA as a first line anti-TNF drug with available baseline BASDAI and CRP were included in the analysis. To get reimbursement for anti-TNF therapy, all AS patients had to have high baseline disease activity (defined as BASDAI >4, and CRP > 10 mg/l). LDA was defined as BASDAI < 4 and CRP <5 mg/l. Pre-specifed sensitivity analyses were conducted in patients with pure axial, and combined axial+peripheral forms of AS.
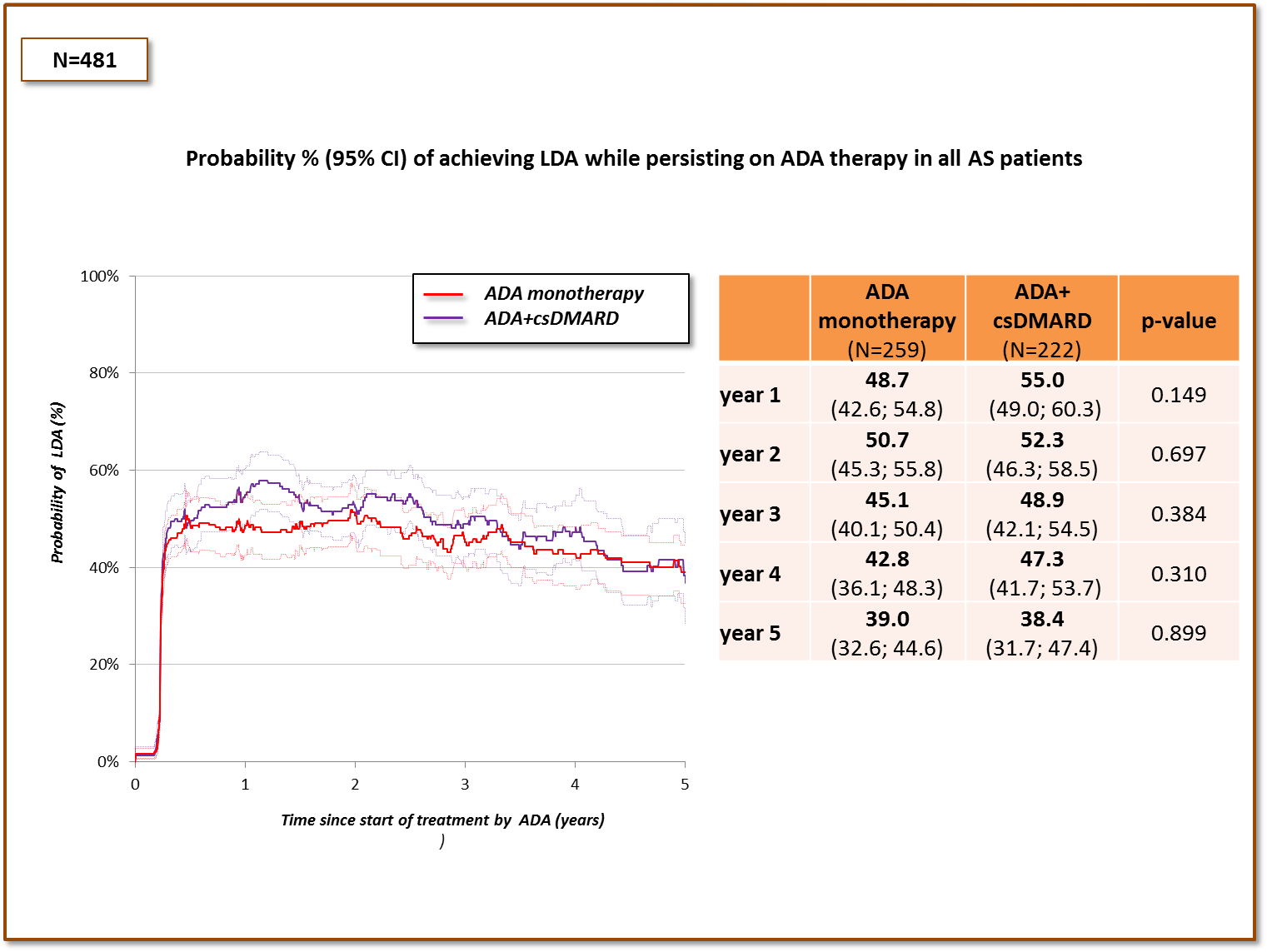
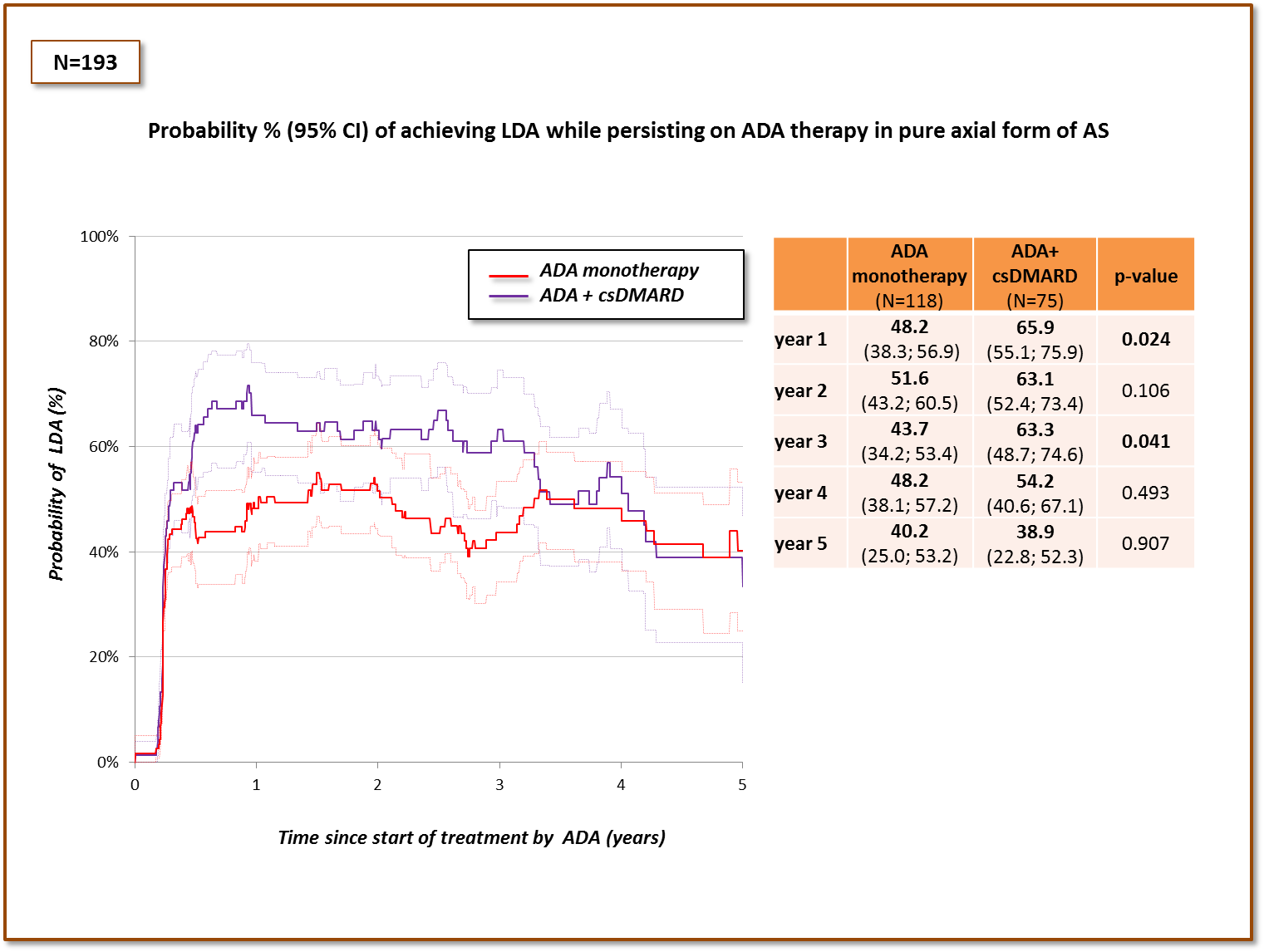
Results: Data for 481 patients were available for the analysis (table). There was no difference in the primary outcome either in the total cohort (figure 1), or in the subset of patients with combined axial and peripheral involvement. In patients with pure axial form of AS, higher probability of achieving LDA while persisting on ADA therapy was observed in some, but not all time-points of follow-up (figure 2). Patients on co-therapy with methotrexate (n=55) fared similarly as those on sulfasalazine (n=141).
Conclusion: Co-therapy with csDMARDs did not increase the overall probability of reaching LDA and drug survival on ADA in AS patients.
Acknowledgements: This work was supported by project of MHCR for conceptual development of research organization 023728.
Table 1 – baseline characteristics
|
ADA monotherapy (N=259) |
ADA+ csDMARD (N=222) |
p-value |
|
|
Female (%) |
24.3 |
33.3 |
0.029 |
|
Age (years) |
39 (10) |
39 (11) |
0.513 |
|
Disease duration (years) |
9,6 (9,4) |
7,7 (7,6) |
0.088 |
|
Pure axial form n(%) |
118 (45.6%) |
75 (33.8) |
0.009 |
|
Axial and peripheral form n(%) |
141 (54.4%) |
147 (66.2) |
|
|
CRP (mg/l) |
23,2 (20,2) |
28,2 (26,7) |
0.111 |
|
BASDAI |
6,1 (1,7) |
6,3 (1,8) |
0.160 |
|
HAQ |
1,1 (0,5) |
1,1 (0,5) |
0.692 |
Data are mean (SD) if not stated otherwise
Figure 1
Figure 2
Disclosure:
K. Pavelka,
None;
J. Zavada,
None;
M. Fojtikova,
None;
S. Forejtova,
None;
K. Hejduk,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-effect-of-co-medication-with-conventional-synthetic-csdmards-on-achieving-low-disease-activity-while-persisting-on-adalimumab-therapy-in-patients-with-ankylosing-spondylitis-axial-spondylarthri/