Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Biosimilars, biopharmaceuticals assessed by regulatory agencies to have efficacy and safety similar to their reference products, were introduced to the UK market in February 2015 for rheumatoid arthritis (RA). Switching patients from originator to biosimilars could result in significant costs savings to the NHS but real world data about efficacy and safety of such a switch are lacking. This analysis describes the characteristics and initial follow-up of RA patients switching from the originator to biosimilars participating in the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis (BSRBR-RA).
Methods: Since 03/08/2015, the BSRBR-RA has captured data on patients starting biosimilars available in the UK: infliximab (Inflectra and Remsima) and later in May 2016, etanercept (Benepali). At biosimilar start, information is captured from the hospital including demographic and clinical data, previous biologic exposure and, if switching therapies, the reason for switch (as a tick box and free text). Follow-up data (disease activity, occurrence of adverse events and changes to treatment) are captured 6-monthly for 3 years and annually thereafter. Descriptive data are presented.
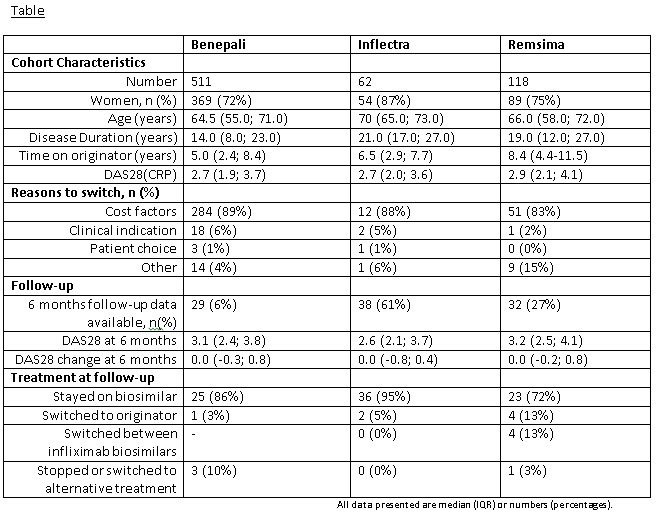
Results: To 10/05/2017, 1,165 patients were recruited at point of starting a biosimilar. Of these, over half (59%, 691/1,165) switched directly from the originator product (74% Benepali, 9% Inflectra, 17% Remsima (table). By far the most frequent reason cited to switch was cost. These patients switched from originator after a median (IQR) time of 5.3 (2.7-8.8) years and the majority had low disease activity (median DAS28 2.7 (IQR 2.0-3.8). To date, follow-up data are available in 99 patients (61% Inflectra, 27% Remsima, 6% Benapali), primarily in those receiving infliximab biosimilars. After 6 months, 85% of these patients stayed on the baseline biosimilar, 7% switched back to their originator, 4% switched between infliximab biosimilars, 3% stopped treatment with a biosimilar and 1% switched to a biologic other than the originator. Reasons for switching back to the originator were inefficacy (n=3) and adverse events (n=2) with two reasons missing. A deterioration in DAS28 of >1.2 after 6 months was experienced by 17% (13/78) of patients. Only one serious adverse event (drug hypersensitivity reaction) was reported in a Remsima patient.
Conclusion: In the UK, RA patients are actively switched from originator to biosimilars mostly for cost reasons. Limited short-term follow up data seem to indicate a high retention on biosimilars, yet a minority of patients had a clinically significant deterioration of their disease activity and some were reported to switch back to the originator already after 6 months of treatment. Data capture will continue and updated reports from the BSRBR-RA will build on these early findings.
To cite this abstract in AMA style:
De Cock D, Kearsley-Fleet L, Watson K, Hyrich KL. Switching from RA Originator to Biosimilar in Routine Clinical Care: Early Data from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/switching-from-ra-originator-to-biosimilar-in-routine-clinical-care-early-data-from-the-british-society-for-rheumatology-biologics-register-for-rheumatoid-arthritis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/switching-from-ra-originator-to-biosimilar-in-routine-clinical-care-early-data-from-the-british-society-for-rheumatology-biologics-register-for-rheumatoid-arthritis/