Session Information
Date: Sunday, November 8, 2015
Title: Rheumatoid Arthritis-Small Molecules, Biologics and Gene Therapy I: Biologics
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: The investigational drug
sarilumab is a human mAb directed against the IL-6 receptor. In previous
studies, sarilumab + MTX demonstrated efficacy in patients (pts) with
moderate-to-severe RA and was generally well tolerated.1 Sarilumab
and tocilizumab have the same mechanism of action. Two studies, Study 1309 (NCT02097524)
and ASCERTAIN (NCT01768572), described
the safety and tolerability of sarilumab and tocilizumab in adults with RA.
Methods: In the 6-wk Study 1309 (N=101), adult
RA pts on background MTX were randomized 1:1:1:1 to receive a single dose of sarilumab
150 mg subcutaneously (SC), sarilumab 200 mg SC, tocilizumab 4 mg/kg intravenously
(IV), or tocilizumab 8 mg/kg IV. In the 24-wk ASCERTAIN study (N=202), RA pts
on background DMARDs with inadequate response to or intolerant of TNF
antagonists were randomized 1:1:2 to sarilumab 150 or 200 mg SC every 2 wks or
tocilizumab every 4 wks starting at 4 mg/kg with an increase to 8 mg/kg if
needed, based on clinical response.
Results: Study 1309 and
ASCERTAIN enrolled RA patients who were predominantly female (>80%), with a mean
age of 55 and 52 yrs, respectively. In ASCERTAIN, 61% of pts on tocilizumab increased
their dose from 4 to 8 mg/kg during the treatment period; 42% of pts increased
their dose at wk 4. Incidence of treatment-emergent adverse events (TEAEs) was similar
for the sarilumab and tocilizumab groups in each study (Table). Upper
respiratory tract infections and neutropenia were among the most frequently
reported TEAEs. Laboratory changes in both studies included increases in lipids
and transaminases and decreases in neutrophil counts. Mean changes in absolute
neutrophil count (ANC) in the sarilumab groups were within the ranges observed
in tocilizumab groups. There was no association between decreased ANC and
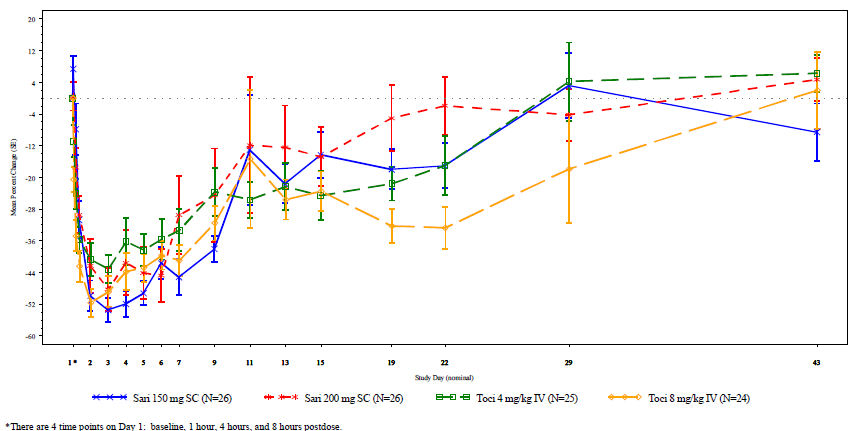
incidence of infection. Study 1309 demonstrated that time to onset for
decreased ANC and magnitude of decrease were comparable across the sarilumab
and tocilizumab groups for all doses (Figure).
Conclusion: Overall, there was no
clinically meaningful difference between the treatment groups with regards to
clinical adverse events. Laboratory changes noted in the sarilumab
groups were within the same range as those noted in the tocilizumab groups. Differences
in incidence of ANC <1.0 Giga/L between the sarilumab and tocilizumab groups
in ASCERTAIN may reflect differences in dosing interval and sampling schedule.
1. Genovese et al. Arthritis Rheumatol. 2015;67:1424-1437.
|
Table. Overview of Adverse Events and Laboratory Parameters in Study 1309 and ASCERTAIN |
|||||||
|
|
Study 1309 |
ASCERTAIN |
|||||
|
|
Patients, n (%) |
Patients, n (%) |
|||||
|
|
Tocilizumab IV 4 mg/kg (n=25) |
Tocilizumab IV 8 mg/kg (n=24) |
Sarilumab SC 150 mg (n=26) |
Sarilumab SC 200 mg (n=26) |
Tocilizumab IV 4 mg/kg (n=102) |
Sarilumab SC 150 mg (n=49) |
Sarilumab SC 200 mg (n=51) |
|
Overview |
|||||||
|
TEAEs |
8 (32%) |
12 (50%) |
10 (39%) |
12 (46%) |
68 (67%) |
33 (67%) |
36 (71%) |
|
SAEs |
0 |
1 (4%) |
0 |
0 |
7 (7%) |
1 (2%) |
3 (6%) |
|
Serious infections |
0 |
0 |
0 |
0 |
2 (2%) |
0 |
1 (2%) |
|
TEAEs leading to death |
0 |
0 |
0 |
0 |
1 (1%) |
0 |
0 |
|
Laboratory parameters |
|||||||
|
Absolute neutrophil countsa |
|
|
|
|
|
|
|
|
<1.0 Giga/L |
3 (12%) |
6 (25%) |
4 (15%) |
7 (27%) |
1 (1%) |
3 (6%) |
5 (10%) |
|
ALTb |
|
|
|
|
|
|
|
|
>3 times ULN |
0 |
2 (8%) |
0 |
1 (4%) |
3 (3%) |
2 (4%) |
3 (6%) |
|
Total cholesterolc |
|
|
|
|
|
|
|
|
≥6.2 mmol/L |
9 (36%) |
6 (25%) |
9 (35%) |
11 (42%) |
50 (50%) |
27 (56%) |
23 (45%) |
|
ALT, alanine aminotransferase; IV, intravenously; SAE, serious adverse event; SC, subcutaneously; TEAE, treatment-emergent adverse event; ULN, upper limit of normal. a1309: 17 post-baseline ANC sampling time points over 43 days; ASCERTAIN: 12 post-baseline ANC sampling time points over 24 weeks. b1309: 5 post-baseline LFT sampling time points over 43 days; ASCERTAIN: 9 post-baseline LFT sampling time points over 24 weeks. c1309: 2 post-baseline lipid sampling time points over 43 days; ASCERTAIN: 5 post-baseline lipid sampling time points over 24 weeks. |
|||||||
Figure. Mean percent change from baseline in ANC by treatment and visit.
ANC, absolute neutrophil count; IV, intravenously; SC, subcutaneously.
To cite this abstract in AMA style:
Emery P, Rondon J, Garg A, van Hoogstraten H, Graham N, Liu M, Parrino J, Spindler AJ, Liu N. Safety and Tolerability of Subcutaneous Sarilumab Compared to Intravenous Tocilizumab in Patients with RA [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/safety-and-tolerability-of-subcutaneous-sarilumab-compared-to-intravenous-tocilizumab-in-patients-with-ra/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-tolerability-of-subcutaneous-sarilumab-compared-to-intravenous-tocilizumab-in-patients-with-ra/