Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The biological disease-modifying anti-rheumatic drugs (bDMARDs) should be used for the treatment of rheumatoid arthritis (RA) in combination with conventional synthetic DMARDs (csDMARDs). However a significant proportion of patients receive bDMARDs in monotherapy.
Methods: Baseline demographic data and efficacy parameters of the first bDMARD treatment of RA patients initiating bDMARD monotherapy (MONO) or combination (COMBI) therapy between 2007 and 2012 were retrieved from the national registry ATTRA. ATTRA is a centralized prospective computerized registry of patients receiving bDMARD therapy collecting data on efficacy, safety and quality of life of all patients treated with bDMARDs. bDMARD therapy was indicated after failure of at least 1 csDMARD (DAS28 ≥5.1).
Results: 1378 patients initiated bDMARD treatment after 2007, prospective data regarding disease activity were available for 924 of them and this subgroup was further analyzed. The 114 patients (12%) who were started on bDMARD monotherapy were older (58 versus 52 years; P<0.001) with longer disease duration (10.1 versus 6.8 years; P=0.001) and more failed csDMARDs in the past (4 versus 3; P=0.002). The baseline DAS28 scores were similar in both groups (5.9 versus 5.8; P=0.083). The most commonly used first line bDMARDs in both groups were antiTNFα drugs (93.9% vs 94.4%; p=0.8). 61.4% MONO patients remained on the same therapy after 12 months, further 21.1% had a csDMARD added to their initial bDMARD. 75.3% COMBI patients remained on the original therapy, 9.7% more were on monotherapy with the first bDMARD. Remission (DAS28<2.6) was reached by 37.8% MONO patients and 41.6% COMBI patients after 12 months. Longitudinal binary logistic regression model was used to calculate the likelihood of reaching remission for both groups at various time points .
|
M o n t h |
|
Crude estimate |
Adjusted estimate* |
||
|
OR (95% IS) |
P value |
OR (95% IS) |
P value |
||
|
3 |
COMBI |
reference |
reference |
||
|
MONO |
0.59 (0.35;0.98) |
0,041 |
0.60 (0.36;1.02) |
0.059 |
|
|
6 |
COMBI |
reference |
reference |
||
|
MONO |
0.60 (0.37;0.98) |
0,040 |
0.61 (0.37;1.01) |
0.055 |
|
|
12 |
COMBI |
reference |
reference |
||
|
MONO |
0.68 (0.44;1.07) |
0,093 |
0.67 (0.42;1.07) |
0.093 |
|
*Adjusted for gender, age, disease duration, number of previous csDMARDs, glucocorticoid use and baseline DAS28.
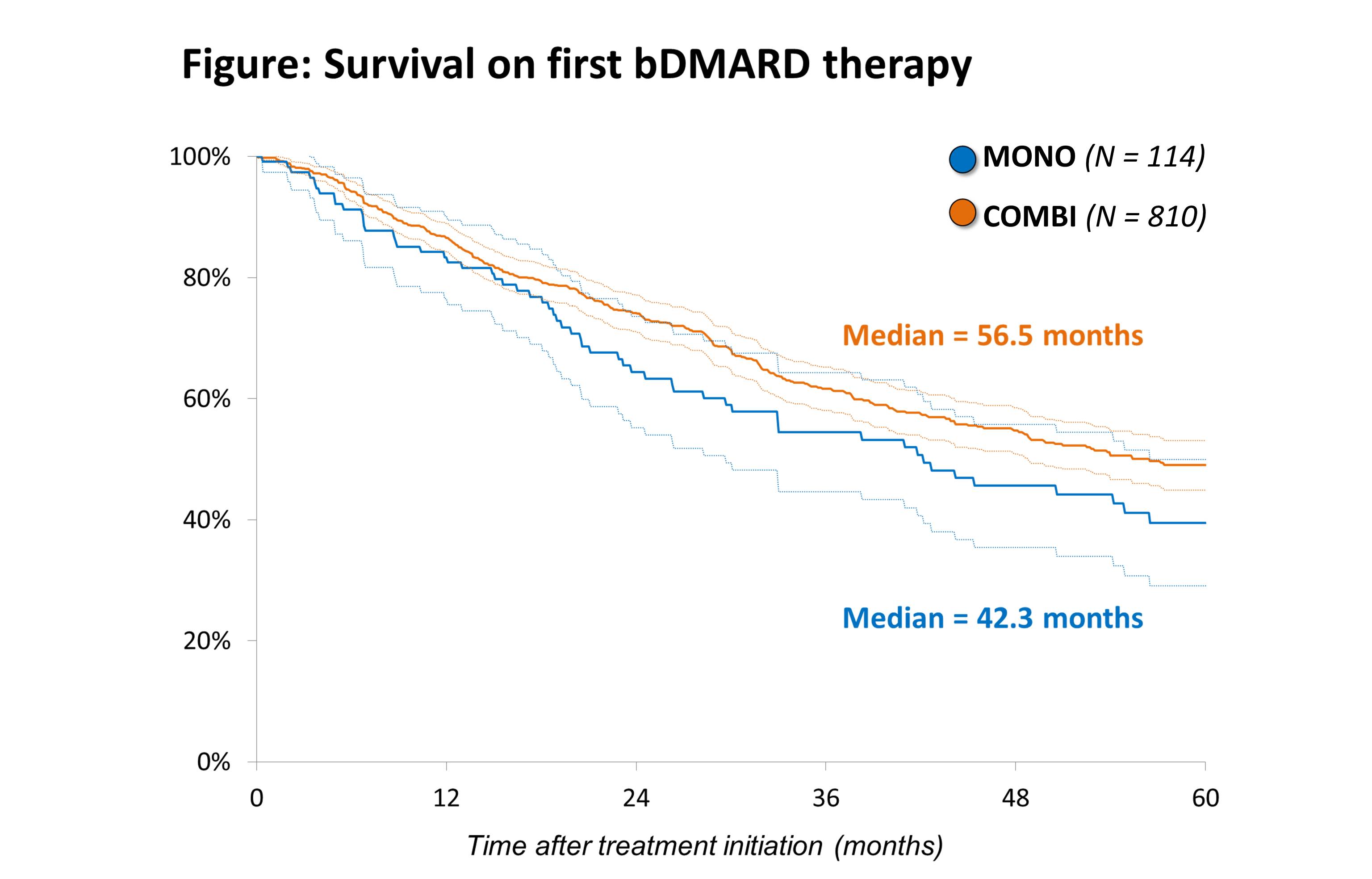
The median survival on the first bDMARD was 42.3 months in the MONO and 56.5 months in the COMBI group (HR 1.29; P=0.073) (Figure).
Conclusion: The results of this observational study suggest that bDMARD efficacy and retention is lower when used as monotherapy, however most observed differences did not reach statistical significance.
Acknowledgements: Supported by project 00023728 from MH CR
Disclosure:
H. F. Mann,
None;
S. Forejtova,
None;
K. Jarosova,
None;
L. Senolt,
None;
J. Zavada,
None;
M. Uher,
None;
K. Hejduk,
None;
K. Pavelka,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/efficacy-of-first-line-biological-monotherapy-in-ra-data-from-the-czech-registry-attra/