Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: This retrospective healthcare claims analysis examined treatment patterns of innovator infliximab (IFX) and biosimilar infliximab (CT-P13) in a Turkish rheumatologic disease population after CT-P13 availability in July, 2014.
Methods: Adult patients (pts) with ≥1 diagnosis code (ICD-10-CM) for rheumatoid arthritis (RA) were identified in a national Turkish healthcare database during the study period (01DEC2010-01DEC2015). Eligible pts had continuous medical/pharmacy enrollment ≥12 months before and ≥6 months after IFX or CT-P13 initiation (index date). Patients were naïve to IFX or CT-P13 (i.e. had no IFX or CT-P13 within 12 months before the index date). Demographics, concomitant diseases and medications, and treatment patterns, eg., dose, interval, discontinuation, and switch were summarized. Confirmed discontinuation was defined as a switch to another biologic medication or the absence of an index biologic claim for ≥120 days without censoring.
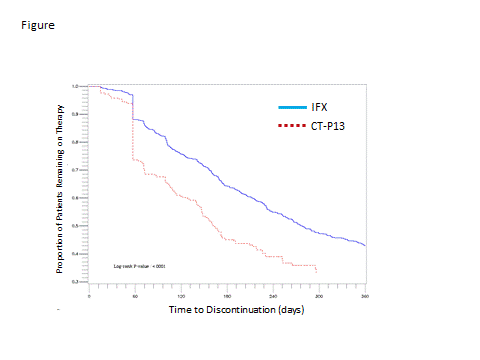
Results: Key results are shown in the Table. A total of 1044 patients initiated either medication. The majority (80%; n=831) initiated IFX. The IFX cohort had a mean age of 42 years; 56% were women and mean follow up was 12 months. The CT-P13 cohort consisted of 213 pts with mean age of 43 years; 58% women; and mean follow up of 9 months. Approximately one-third of pts in each cohort had a concomitant diagnosis of ankylosing spondylitis (AS; TABLE). Other concomitant diseases and medications appeared balanced between cohorts. Pts in the IFX cohort had an average of 5.2 infusions and mean dose of 4.7 vials per infusion approximately every 8 weeks. Pts in the CT-P13 cohort had an average of 3.6 doses and mean dose of 5.8 vials per dispensing approximately 9 weeks apart. A confirmed discontinuation occurred in 55% of the IFX cohort; driven in part by switching. 24% of IFX pts had ≥ 1 biologic switch with 8% initially switching to CT-P13. Time to any discontinuation or censoring of IFX is shown in the Figure and Table. In the CT-P13 cohort, a confirmed discontinuation was observed in 63%; 31% switched to another biologic therapy; and 20% initially switched to IFX. Time to any discontinuation or censoring of CT-P13 is shown in Figure and Table.
Conclusion: These findings in a single country indicate that real world utilization patterns may differ between innovator IFX and CT-P13, with predominantly more patients initiating IFX; greater overall CT-P13 discontinuation and a higher proportion of patients switching from CT-P13 to IFX. Further studies are needed to understand the reasons for these observed differences. Table
|
Innovator IFX Cohort |
CT-P13Cohort |
|||||
|
(N= 831) |
(N=213 ) |
|||||
|
N/Mean |
%/SD |
N/Mean |
%/SD |
|||
| Age (Mean) |
42 |
13 |
43 |
12 |
||
| Gender | ||||||
|
Female |
465 |
56% |
124 |
58 % |
||
| Average Length of Follow up Period ( in Months) |
12 |
3 |
9 |
2 |
||
|
Concomitant Disease During Baseline Period |
|
|
|
|
||
|
Ankylosing Spondylitis |
230 |
28% |
70 |
33% |
||
|
Psoriatic Arthritis |
130 |
16% |
24 |
11% |
||
|
Crohn’s Disease |
65 |
8% |
11 |
5% |
||
|
Ulcerative Colitis |
64 |
8% |
10 |
5% |
||
|
Concomitant RA-Medications During Follow up Period |
||||||
|
Methotrexate |
253 |
30% |
69 |
32% |
||
|
Sulfasalazine |
147 |
18% |
46 |
22% |
||
|
Dosing Characteristics |
|
|
|
|
||
| Average # of doses within follow up period |
5.2 |
2.6 |
3.6 |
1.8 |
||
| Mean # of weeks between doses |
8.2 |
4.2 |
9.0 |
4.7 |
||
|
Mean # of days between 1st and 2nd dose |
38 |
37 |
50 |
41 |
||
|
Mean # of days between 2nd and 3rd dose |
53 |
33 |
60 |
38 |
||
|
Mean # of days between 3rd and 4th dose |
65 |
34 |
67 |
31 |
||
|
Switching |
|
|
|
|
||
|
# and % of patients with ≥1 switch |
203 |
24% |
66 |
31% |
||
|
# of patient switches between Infliximab Types |
|
|
|
|
||
|
Switch to CT-P13 |
64 |
8% |
||||
|
Switch to Innovator Infliximab |
42 |
20% |
||||
|
Discontinuations |
|
|
|
|||
|
# of Patients Confirmed to Have Discontinued |
453 |
55% |
134 |
63% |
||
|
Time to confirmed discontinuation (days) |
155 |
93 |
107 |
66 |
||
|
Time to any discontinuation or censoring (days): |
239 |
130 |
169 |
102 |
||
To cite this abstract in AMA style:
Yazici Y, Xie L, Ogbomo A, Parenti D, Goyal K, Teeple A, Ellis LA, Simsek I. A Descriptive Analysis of Real-World Treatment Patterns of Innovator Infliximab (Remicade) and Biosimilar Infliximab in a Treatment NaïVe Turkish Rheumatologic Disease Population [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/a-descriptive-analysis-of-real-world-treatment-patterns-of-innovator-infliximab-remicade-and-biosimilar-infliximab-in-a-treatment-naive-turkish-rheumatologic-disease-population/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-descriptive-analysis-of-real-world-treatment-patterns-of-innovator-infliximab-remicade-and-biosimilar-infliximab-in-a-treatment-naive-turkish-rheumatologic-disease-population/